New Era of Biomarker-driven Clinical Trials & Regulatory Approvals in US & China
Date and time
Location
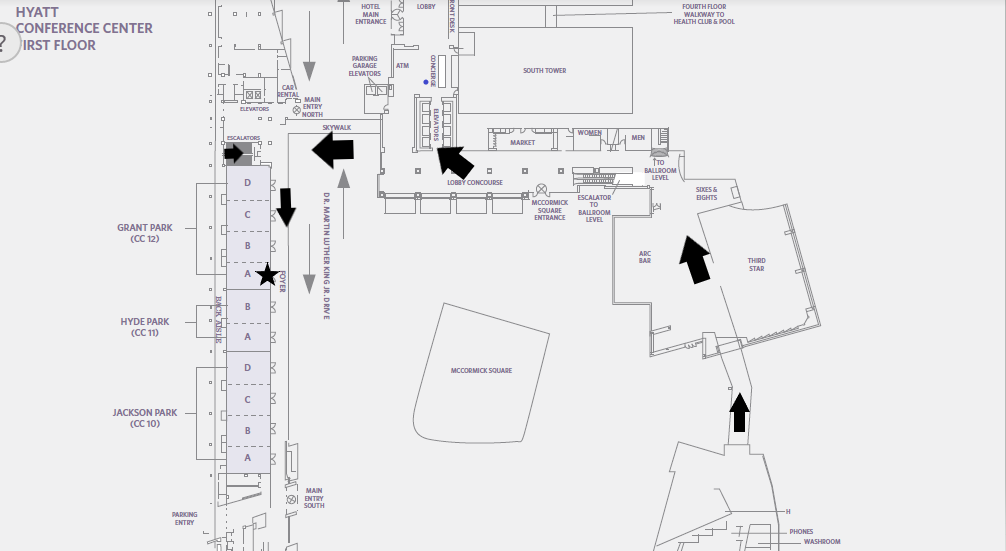
Hyatt Regency McCormick Place
2233 South Doctor Martin Luther King Junior Drive Room: Grant Park A Chicago, IL 60616Description
New Era of Biomarker-driven Clinical Trials and Regulatory Approvals in US and China
This educational symposium will provide a forward-looking perspective of how biopharma companies could leverage innovations in biomarkers and regulatory initiatives to enable precision clinical trials and concurrent NDA submissions in US and China.
Recently, both FDA and NMPA encouraged biomarker-driven precision clinical trials and cross-border NDAs, even with clinical data generated solely in one region such as China, US, or EU. This has sparked strong interests for Chinese oncology pharmaceuticals to enter the Western market. Meanwhile, China is becoming the first country to get new drugs approved, ahead of US and EU, further driving the need for global clinical trials.
A panel of industry leaders and regulatory experts will discuss how technology and regulatory innovation will reshape the global drug development landscape.
Agenda:
6:00pm - 6:30pm Reception (food and drinks will be served)
6:30pm - 7:30pm Welcome and Presentations
- Liquid biopsy and comprehensive biomarker solutions for global clinical trials - Shidong Jia, CEO, Predicine
- Cross-border clinical trials and New Drug Applications in U.S and China - Dan Zhang, Executive Chairman, Fountain Medical Development
7:30pm - 8:00pm Networking
How to get to Grand Park A meeting room from McCormick Place: